4a. What is Carbon Dioxide?
What is Carbon Dioxide?
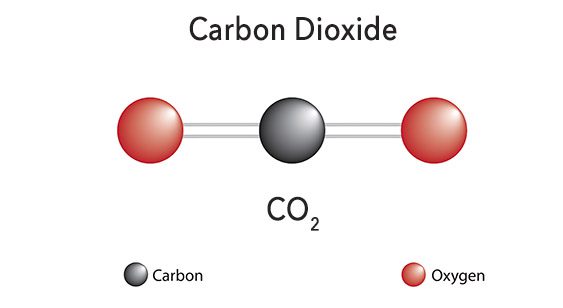
As you learned in Lesson 3, carbon dioxide (CO2) is a molecule made up of one carbon atom bonded to two oxygen molecules. CO2 occurs naturally and is a colorless, odorless gas at normal temperatures and pressures. When cooled down to extreme temperatures, the gas turns into a white solid, commonly known as dry ice. CO2 can also be pressurized and condensed into a liquid.
Uses of Carbon Dioxide?
While carbon dioxide is a major focus of pollution reduction, it also has many useful applications. Tap or click the cards to learn more about the uses of CO2.

Pressurized liquid CO2 is used in fire extinguishers. Spraying flames with CO2 cuts off oxygen from the flames and puts out the fire.

Drinks are carbonated by dissolving CO2 into a liquid and pressurizing it. When the drink is opened, the pressure is released and the CO2 begins to bubble out of the drink.

The amount of oil recovered from drilling can be increased by injecting CO2 into the oil recovery zone and using it to push the oil closer to the well.

Blocks of dry ice, or solid CO2, can be shipped with foods to keep them chilled and dry. Unlike regular ice which melts into a liquid, solid CO2 turns into a gas at normal temperatures.

Pressurized CO2 can be used as a propellant in spray cans.
